What quantity,in moles,of hydrogen is consumed when 179.6 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ? H2(g) + 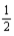 O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
O2(g) → H2O(l) ; ΔrH° = -285.8 kJ/mol-rxn
Definitions:
Personal Responsibility
The idea that individuals are accountable for their actions and decisions, and must take ownership of the consequences that come from those actions and decisions.
Successes
Achievements of an aim or goal, generally bringing a sense of accomplishment and pride to the individual or group involved.
Failures
Instances or events when desired goals or expectations are not met, often viewed as opportunities for learning and improvement.
Overconfident
Characterized by an overly strong belief in one's abilities or outcomes, often more than is warranted by the reality.
Q10: Properties,such as color and density,which can be
Q12: Point D on the phase diagram is
Q24: How long will it take 10.0 mL
Q32: Employees in the Sales department need to
Q39: The SI unit of temperature is the
Q40: What volume of gaseous silane,SiH<sub>4</sub>,has the same
Q44: Many homes are heated using natural gas.The
Q47: The complete electron configuration of tin is
Q68: What is the charge on the sodium
Q83: Gaseous chlorine is held in two separate