When 50.0 mL of 1.60 M of HCl(aq) is combined with 50.0 mL of 1.70 M of NaOH(aq) in a coffee-cup calorimeter,the temperature of the solution increases by 10.7°C.What is the change in enthalpy for this balanced reaction? HCl(aq) + NaOH(aq) → NaCl(aq) + H2O( 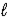 )
)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g⋅°C.
Definitions:
Accommodation
Adjusting one's language or behavior to meet the needs or preferences of others, often in communication contexts.
Disinhibition Effect
The phenomenon where people exhibit uninhibited behavior online that they would not display in face-to-face interactions.
Collaboration
Working together cooperatively towards a common goal or project, often involving shared responsibility and contributions.
Flaming
Sending an overly negative online message that personally attacks another person.
Q18: The percent yield of a chemical reaction
Q41: Windows Server 2012 can be installed on
Q42: The volume of space occupied by a
Q49: Naphthalene,a hydrocarbon,has an approximate molar mass of
Q50: Equal volumes of propane,C<sub>3</sub>H<sub>8</sub>,and carbon monoxide,CO,at the
Q57: The nitrogen atom in the cyanide ion,CN<sup>-</sup>,is
Q62: What is the hybridization of each carbon
Q65: If the energy of 1.00 mole of
Q67: List all the intermolecular forces present in
Q89: What is the common name of the