When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction,98.8 kJ of energy is absorbed. Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 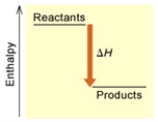
 (A)
(A)
(B)
Is the reaction endothermic or exothermic,and which of the enthalpy diagrams above
Represents this reaction?
Definitions:
Budget
An estimate of income and expenditure for a set period of time, often used as a plan for managing finances.
Organizations
Entities composed of individuals and resources, structured to achieve specific goals through a collective effort.
Bureaucratic Control System
A formal system of organization based on clearly defined hierarchical levels and roles, focused on following rules and procedures to manage operations.
Q1: Two important allotropes of phosphorus are white
Q16: Atomic orbitals combine most effectively to form
Q20: Refer to Diagram 9-1.According to molecular orbital
Q34: What does the ping message "Destination net
Q39: The procedure by which electrons are assigned
Q39: What is the hybridization of the nitrogen
Q41: For which one of the following elements
Q48: Benzene,C<sub>6</sub>H<sub>6</sub>,consists of a six member ring of
Q65: If the energy of 1.00 mole of
Q75: A vessel with a volume of 17.3