Aspirin (C9H8O4) is produced by the reaction of salicylic acid (M = 138.1 g/mol) and acetic anhydride (M = 102.1 g/mol) . C7H6O3(s) + C4H6O3( 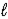 ) → C9H8O4(s) + C2H4O2(
) → C9H8O4(s) + C2H4O2( 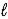 )
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol) can theoretically be obtained?
Definitions:
Trust Accounts
Financial accounts held by a trustee for the benefit of a third party according to the terms of a trust agreement.
Gross Earnings
The total amount of money earned by an individual or company before any deductions such as taxes or benefits.
Income Taxes
Taxes levied by a government directly on income, both earned (salaries, wages, commissions) and unearned (dividends, interest, rents).
Petty Cash Fund
A small amount of cash on hand used for minor, incidental expenses within a business or organization.
Q4: In valence bond theory,each sigma bond in
Q9: What is the total number of subshells
Q10: How do you map a shared folder
Q13: What is the hybridization of carbon atoms
Q13: Which of the following virtualization products uses
Q26: The elements in groups 1A-8A are known
Q32: Zn reacts with hydrochloric acid. Zn(s)+ 2
Q45: What is the change in internal energy
Q46: SF<sub>3</sub><sup>+</sup>,has a tetrahedral electron-pair geometry and a
Q68: What is the charge on the sodium