If 0.1800 g of impure soda ash (Na2CO3) is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash? Na2CO3(aq) + 2 HCl(aq) → 2 NaCl(aq) + H2O( 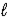 ) + CO2(g)
) + CO2(g)
Definitions:
Employee Salaries And Wages
The compensation paid to employees for their labor, including both hourly wages and fixed salaries.
Planning Budget
A budget created at the beginning of a period, based on forecasted levels of activity, to guide business operations and financial planning.
Total Expenses
encompass all costs incurred by a business or individual within a specified period, including operational, financial, and non-operational expenses.
Planning Budget
An estimate of income and expenditure for a set period, often used to guide financial decision-making and control.
Q4: A mass of 10 g of table
Q4: What directory service protocol is used by
Q5: The central atom in SF<sub>6</sub> is surrounded
Q24: What is the de Broglie wavelength of
Q41: What is the difference between the two
Q42: Which of the following elements belongs to
Q43: If a cordless phone operates at a
Q59: Write a balanced chemical equation for the
Q63: For which of the following transitions would
Q78: Which group of the periodic table of