Which one of the statements below is false concerning the following reaction: NH3(g) + H2O 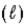
 NH4+(aq) + OH−(aq)
NH4+(aq) + OH−(aq)
Definitions:
Average Total Cost
The total cost of production (fixed and variable costs combined) divided by the total quantity produced, indicating the cost per unit of product.
Marginal Revenue
The additional income received from selling one more unit of a good or service.
Industry Supply Curve
A graphical representation showing the total quantity of a good or service that producers in an industry are willing and able to supply at different price levels.
Marginal Cost Curves
A graphical representation showing how the cost of producing one more unit of a good varies with the quantity of the good produced.
Q9: Convert 0.390 ng to milligrams and express
Q18: A metal oxide forms when potassium reacts
Q26: In the reaction below,which species is oxidized?
Q30: What is another name for a RAID
Q36: Which of the following is a protocol
Q37: A student uses a balance to determine
Q59: If a gallon (3.78 L)of latex paint
Q59: Which of the labeled carbon atoms (C<sub>1</sub>-C<sub>4</sub>)is/are
Q65: What base results when K<sub>2</sub>O reacts with
Q73: Which ground-state electron configuration is incorrect?<br>A) Br: