Use the figure below to answer the following questions.
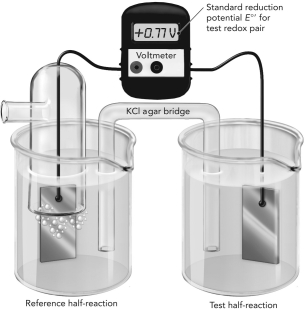 A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
A. The electrochemical cell shown here is measuring the reduction potential of Fe2+/Fe3+ using a reference hydrogen half reaction. Write each half reaction on the figure and show the flow of electrons.
B. Do these oxidants have a higher or lower affinity for electrons compared with H+? Explain how you know this using information from the experiment.
Definitions:
Q9: Which of the following metabolites has carbon
Q41: Which molecules function as electron acceptors in
Q43: Which technique ionizes polypeptides by embedding the
Q45: What molecule activates the pentose phosphate pathway?<br>A)
Q59: Define the term zymogen.
Q66: How is penicillin inactivated by penicillin-resistant
Q70: A patient seeks medical attention for ulcerous
Q73: The following peptides are separated using an
Q79: Which pathway is opposite to gluconeogenesis?<br>A) TCA
Q80: Polyclonal antibodies are<br>A) derived from B cells