A voltaic cell is constructed with two silver-silver chloride electrodes, where the half-reaction is AgCl(s) + 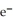 → Ag(s) +
→ Ag(s) + 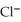 (aq) E° = +0.222 V
(aq) E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0557 mol L-1 and 5.31 mol L-1, respectively. The cell emf is ________ V.
Definitions:
Fecal Occult Blood
This is a clinical test for detecting hidden blood in the stool, an indicator that may suggest gastrointestinal bleeding.
Blood Specimen
A sample of blood taken for testing or analysis to diagnose, monitor, or treat various health conditions.
Sputum
A mixture of saliva and mucus coughed up from the respiratory tract, especially from the lungs, used for diagnosing respiratory diseases.
Angiography
A medical imaging technique used to visualize the inside of blood vessels and organs of the body, specifically arteries, veins, and the heart chambers.
Q3: Determine the molar solubility of AgBr in
Q11: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt=" A)amine B)ketone C)aldehyde
Q45: Calculate Δ<sub>r</sub>G at 298 K under the
Q67: Identify hybridization of carbon atoms numbered 1-6
Q69: The infrared spectrum of a compound with
Q74: Which of the following is the strongest
Q74: Which of the following is a Lewis
Q88: Determine the cell notation for the redox
Q97: Determine the absolute configuration of the molecule
Q134: Determine the temperature of a reaction if