A voltaic cell is constructed with two silver-silver chloride electrodes, where the half-reaction is AgCl(s) + 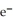 → Ag(s) +
→ Ag(s) + 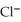 (aq) E° = +0.222 V
(aq) E° = +0.222 V
The concentrations of chloride ion in the two compartments are 0.0557 mol L-1 and 5.31 mol L-1, respectively. The cell emf is ________ V.
Definitions:
Initial Encounter
A term denoting the very first consultation or treatment session for a new health problem or diagnosis.
Appendicular Skeleton
Refers to the portion of the skeleton that includes the bones of the arms, legs, pelvis, and shoulder girdle.
Clavicle
A long bone that serves as a strut between the shoulder blade and the sternum or breastbone, commonly known as the collarbone.
Scapula
The scapula, or shoulder blade, is a large, flat, triangular bone located in the upper back on each side of the vertebral column.
Q19: Calculate the concentration of OH⁻ in a
Q29: Draw the Lewis structure for IF<sub>4</sub>⁻ and
Q35: A 1.00 L buffer solution is 0.150
Q42: Calculate the equilibrium constant for the following
Q47: Define the second law of thermodynamics.
Q56: Which of the following is TRUE about
Q59: Which of the following statements is TRUE?<br>A)
Q104: Identify the expression for the solubility product
Q115: Identify the strongest acid.<br>A) H<sub>2</sub>O<br>B) H<sub>2</sub>S<br>C) H<sub>2</sub>Se<br>D)
Q136: Identify the weak diprotic acid.<br>A) HNO<sub>3</sub><br>B) H<sub>3</sub>PO<sub>4</sub><br>C)