A voltaic cell is constructed with two 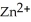 -Zn electrodes, where the half-reaction is
-Zn electrodes, where the half-reaction is 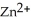 +
+ 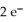 → Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 ×
→ Zn (s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 1.17 × 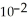 mol L-1 and 8.70 ×
mol L-1 and 8.70 × 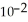 mol L-1, respectively. The cell emf is ________ V.
mol L-1, respectively. The cell emf is ________ V.
Definitions:
Fair Value Method
An accounting approach that assesses and assigns a price to a company's assets and liabilities based on current market value.
Effective Interest Method
A method of calculating the amortized cost of a bond or loan on the basis on an effective interest rate rather than the nominal rate.
Cash Interest
Interest payments made in cash to creditors or bondholders during a specific period.
Trading Securities
Shares or bonds that a company holds with the intention of selling them within a short period for a profit.
Q1: What is the index of hydrogen deficiency
Q6: What is the maximum ratio of conjugate
Q24: What type of lipid is the following
Q26: Find the suitable base for the following
Q32: Determine the identity of the daughter nuclide
Q45: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt=" A)aspartic acid B)lysine
Q73: Consider a reaction that has a negative
Q102: Consider a reaction that has a positive
Q122: What mass of silver can be plated
Q128: Determine Δ<sub>r</sub>G° at 298 K using the