For a given reaction, ΔrH = -19.5 kJ mol-1 and ΔrS = -55.8 J K-1 mol-1. The reaction will have 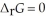 at
at  Assume that ΔrH and ΔrS do not vary with temperature.
Assume that ΔrH and ΔrS do not vary with temperature.
Definitions:
Zero Economic Profits
A situation in perfectly competitive markets where firms earn just enough revenue to cover their total operating costs, leaving no supernormal profit.
Positive Economic Profits
The situation in which a firm's total revenues exceed its total costs, including both explicit and implicit costs, signaling that the firm is doing better than the next best alternative.
Profit
The financial gain realized when the amount earned from a business activity exceeds the expenses, costs, and taxes needed to sustain the activity.
Monopolistically Competitive Firm
A company that operates in a market with many competitors, each offering products that are similar but not identical, allowing for some degree of market power.
Q2: The decomposition of ammonia is 2NH<sub>3</sub>(g) →
Q14: Above what atomic number are there no
Q25: Predict the species that will be reduced
Q58: A 1.0 L buffer solution is 0.250
Q60: Write the nuclear equation for the beta
Q62: Consider the following reaction and its equilibrium
Q63: Determine the identity of the daughter nuclide
Q86: The equilibrium constant is given for one
Q89: What is the autoionization of water?
Q138: The following reaction is exothermic. Which change