Calculate the pH of a solution that is 0.445 mol L-1 in sodium formate (HCOONa) and 0.265 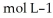 in formic acid (HCOOH) . The
in formic acid (HCOOH) . The 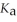 of formic acid is 1.77 ×
of formic acid is 1.77 × 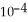 .
.
Definitions:
Historical Cost
The original monetary value of an asset or investment at the time of its acquisition.
Fair Value
Fair Value is a financial term describing the estimated worth of an asset or liability in the marketplace, based on current prices and considering factors like utility and risk.
Asset
Resources owned or controlled by a business that are expected to generate future economic benefits.
Fair Value Principle
Assets and liabilities should be reported at fair value (the price received to sell an asset or settle a liability).
Q21: Determine the [H<sub>3</sub>O⁺] in a 0.265 mol
Q22: Which of the following statements is TRUE?<br>A)
Q34: Calculate Δ<sub>r</sub>G for the evaporation of methanol
Q37: The standard emf for the cell using
Q44: Consider the following reaction at equilibrium. What
Q60: Identify the statement that is TRUE.<br>A) The
Q110: proton<br>A)<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="proton A) e B)
Q113: For which reaction will K<sub>p</sub> = K<sub>c</sub>?<br>A)
Q119: Use the following thermodynamic values to calculate
Q124: Determine the equilibrium constant for the following