Calculate the pH of a solution that is 0.240 mol L-1 in nitrous acid ( 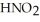 ) and 0.320 mol L-1 in potassium nitrite (
) and 0.320 mol L-1 in potassium nitrite ( 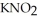 ) . The acid dissociation constant of nitrous acid is 4.50 ×
) . The acid dissociation constant of nitrous acid is 4.50 ×  .
.
Definitions:
Equipment Purchase
The acquisition of machinery or office equipment, usually classified as a capital expense, to be used in the operations of a business.
Land Purchase
An acquisition of real estate property involving a one-time payment or installment payments, considered a long-term asset on the balance sheet.
Net Cash Flow
The difference between a company's cash inflows and outflows within a given period, indicating the company's ability to generate cash.
Operating Activities
Business actions that relate to the day-to-day operations, producing revenue and spending money to operate the business.
Q2: What is the molar solubility of AgCl
Q25: Predict the species that will be reduced
Q39: At a certain temperature the equilibrium constant,
Q50: A reaction with an activation energy of
Q64: Calculate the pH of a buffer that
Q70: Beta decay of <sup>60</sup>Co produces a beta
Q72: Calculate the pH of a solution that
Q75: The following reaction represents what nuclear process?
Q77: Determine the pH in a 0.435 mol
Q102: What are constitutional isomers?