Calculate the percent ionization of nitrous acid in a solution that is 0.210 mol L-1 in nitrous acid 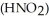 and 0.290 mol L-1 in potassium nitrite (
and 0.290 mol L-1 in potassium nitrite ( 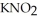 ) . The acid dissociation constant of nitrous acid is
) . The acid dissociation constant of nitrous acid is 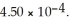
Definitions:
Term Structure
The term structure, often related to interest rates, outlines the relationship between interest rates or yields and different maturity dates for debt securities.
Inflation Premium
The portion of a nominal interest rate that represents compensation for expected future inflation.
Interest Rate Risk
The risk that changes in interest rates will adversely affect the value of an investment, particularly relevant for fixed-income securities.
Q29: Complete the following equation of transmutation. <br><img
Q37: A solution containing AgNO<sub>3</sub> is mixed with
Q62: pH = pK<sub>a2</sub><br>A)equivalence point of a weak
Q62: Calculate the hydronium ion concentration in an
Q73: Which of the following is correct?<br>A) The
Q87: Which of the following is an acid-base
Q87: Write a nuclear equation to describe the
Q91: pH < 7<br>A)equivalence point of a weak
Q103: Give the standard states for a gas,
Q120: Why aren't solids or liquids included in