Calculate the percent ionization of nitrous acid in a solution that is 0.249 mol L-1 in nitrous acid. The acid dissociation constant of nitrous acid is 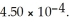
Definitions:
Lung Cancer
A type of cancer that begins in the lungs, characterized by uncontrolled cell growth in tissues of the lung, leading to a variety of respiratory and systemic symptoms.
Smokers
Individuals who inhale the smoke of burning tobacco products, such as cigarettes, cigars, or pipes.
Occupational Hazards
Potential risks or dangers associated with specific jobs or workplace environments that could harm employees.
Bradycardia
A slower than normal heart rate, typically defined as fewer than 60 beats per minute in adults, which can affect blood flow and oxygen to the body.
Q1: The pH of a solution is 2.62
Q24: The first-order decomposition of N<sub>2</sub>O<sub>5</sub> at 328
Q89: Determine the Q of a reaction at
Q96: Determine the [OH<sup>-</sup>] concentration of a 0.116
Q104: Given the following equation, N<sub>2</sub>O(g) + NO<sub>2</sub>(g)
Q114: Determine the missing initial rate for the
Q118: Determine the equilibrium constant for the following
Q131: For the reaction: N<sub>2</sub>(g) + 2O<sub>2</sub>(g) ⇌
Q135: Consider the following reaction: NO(g) + SO<sub>3</sub>(g)
Q135: Calculate the activation energy, E<sub>a</sub>, for a