How many millilitres of 0.120 mol L-1 NaOH are required to titrate 50.0 mL of 0.0998 mol L-1 acetic acid to the equivalence point? Acetic acid is monoprotic. The 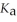 of acetic acid is 1.8 × 10-5.
of acetic acid is 1.8 × 10-5.
Definitions:
Freight Loadings
The process or act of loading cargo for transport by various modes of transportation like trains, ships, or airplanes.
Parallelism
The use of components in sentences that are grammatically the same, or similar in their construction, sound, meaning, or meter.
Morale
The confidence, enthusiasm, and discipline of a person or group at a particular time.
Absenteeism
The habitual non-presence of an individual supposed to be at work, school, or other engagements often without valid reason.
Q18: Calculate the cell potential for the following
Q59: Calculate the mass defect in Fe-56 if
Q75: The following reaction represents what nuclear process?
Q76: What is the pH of a solution
Q80: Use the standard half-cell potentials listed below
Q101: Use the free energies of formation given
Q118: Determine the equilibrium constant for the following
Q138: What does the term amphoteric mean?
Q140: Consider the following reaction at equilibrium. What
Q165: Given the following balanced equation, determine the