What is the molar solubility of Mg(OH) 2 in a basic solution with a pH of 12.50? Ksp for Mg(OH) 2 is 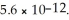
Definitions:
Greatest Density
Refers to the area in a distribution or data set where points are most tightly clustered.
Normally Distributed
Describes a statistical distribution that is symmetric about the mean, showing that data near the mean are more frequent in occurrence than data far from the mean.
Gasoline Prices
The cost per unit of gasoline, which can fluctuate based on factors such as crude oil prices, supply and demand, taxes, and market speculation.
Standard Normal Distribution
A normal distribution with a mean of zero and a standard deviation of one, used in many statistical methods.
Q3: Which of the following statements is TRUE?<br>A)
Q9: What is the pH of a buffer
Q14: Consider the following reaction and its equilibrium
Q27: Use the provided reduction potentials to calculate
Q46: Determine the identity of the daughter nuclide
Q70: Calculate the cell potential for the following
Q99: Identify the expression for the solubility product
Q111: Consider the following reaction: Xe(g) + 2F<sub>2</sub>(g)
Q115: Use Hess's law to calculate Δ<sub>r</sub>G° using
Q165: Given the following balanced equation, determine the