A solution is prepared by dissolving 0.23 mol of formic acid and 0.27 mol of sodium formate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution. The 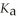 of formic acid is 1.36 × 10-3.
of formic acid is 1.36 × 10-3.
Definitions:
Gain Recognition
The process by which gains from the sale or exchange of assets are reported for tax purposes.
Involuntary Conversion
The forced exchange of an asset for compensation, such as through eminent domain or destruction, where the proceeds may qualify for special tax treatment.
Insurance Company
A business that provides financial protection and compensation for losses in exchange for the payment of premiums.
Basis
The amount of investment in an asset for tax purposes, used to calculate gain or loss upon sale of the asset.
Q20: The splitting of the uranium atom is
Q26: A 100.0 mL sample of 0.20 mol
Q46: In a reaction mixture containing reactants and
Q50: Express the equilibrium constant for the following
Q66: What is the oxidizing agent in the
Q77: Which of the following represents the integrated
Q91: Determine the identity of the daughter nuclide
Q92: Q > 1<br>A)standard state<br>B)equilibrium<br>C)Δ<sub>r</sub>G > Δ<sub>r</sub>G°<br>D)Δ<sub>r</sub>G >
Q128: Calculate the pH of a 0.800 mol
Q132: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,