Calculate the pH of a solution that is 0.445 mol L-1 in sodium formate (HCOONa) and 0.265 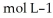 in formic acid (HCOOH) . The
in formic acid (HCOOH) . The 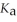 of formic acid is 1.77 ×
of formic acid is 1.77 × 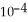 .
.
Definitions:
Accurate Coding
The process of correctly categorizing medical diagnoses, treatments, and procedures using a standardized system for billing and documentation purposes.
Document Management
The employment of computer systems and software applications to archive, administer, and monitor both electronic documents and digital versions of paper-based data.
Forced Entry
The act of entering a property without permission, often by breaking in.
Building Security
Measures and systems implemented to ensure the safety and protection of a building and its occupants from threats such as unauthorized access, theft, and violence.
Q3: Which of the following statements is TRUE?<br>A)
Q5: How many half-lives are required for the
Q19: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q35: Under which of the following conditions would
Q45: Calculate Δ<sub>r</sub>G at 298 K under the
Q56: Determine the equilibrium constant for the following
Q87: Determine the missing initial rate for a
Q99: Determine the pH of a 0.00598 mol
Q118: Can the K<sub>p</sub> and K<sub>c</sub> for a
Q128: Sodium hypochlorite, NaOCl, is the active ingredient