Calculate the percent ionization of nitrous acid in a solution that is 0.210 mol L-1 in nitrous acid 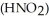 and 0.290 mol L-1 in potassium nitrite (
and 0.290 mol L-1 in potassium nitrite ( 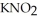 ) . The acid dissociation constant of nitrous acid is
) . The acid dissociation constant of nitrous acid is 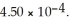
Definitions:
At-Risk
The at-risk rules limit the amount of loss a taxpayer can claim to the amount actually at risk in the investment activity.
Form 6198
An IRS form used by taxpayers to determine the amount of at-risk activities loss that is deductible for the tax year.
At-Risk Amounts
At-Risk Amounts refer to the amount of money an individual could lose in an investment or venture, indicating the level of risk involved in the investment.
At-Risk Amount
The maximum amount of money or other assets that a taxpayer can claim as a deduction or loss from an activity, to the extent of the actual economic risk.
Q6: What is the maximum ratio of conjugate
Q10: The reaction below has a K<sub>c</sub> value
Q17: Identify the pH of normal blood.<br>A) 7.0<br>B)
Q33: Determine the identity of the daughter nuclide
Q74: Consider the following reaction: CH<sub>4</sub>(g) + 2H<sub>2</sub>S(g)
Q85: Express the equilibrium constant for the following
Q101: What is the activation energy for a
Q142: Formic acid (HCOOH, K<sub>a</sub> = 1.8 ×
Q144: Write a balanced reaction for which the
Q150: Lewis acid<br>A)electron pair donor<br>B)produces protons in aqueous