How many millilitres of 0.120 mol L-1 NaOH are required to titrate 50.0 mL of 0.0998 mol L-1 acetic acid to the equivalence point? Acetic acid is monoprotic. The 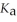 of acetic acid is 1.8 × 10-5.
of acetic acid is 1.8 × 10-5.
Definitions:
Relationship
Pertains to the connections and interactions between a business and its customers, aiming to foster loyalty and long-term engagement.
Demand Curve
A graph that illustrates the relationship between the price of a good or service and the quantity demanded by consumers.
Price and Demand
Describes the relationship between the price of a good or service and the willingness of people to either buy or sell it at that price.
Assumption
A belief or statement taken for granted without proof, often serving as a basis for further reasoning or arguments.
Q1: For which of the following reactions will
Q7: A solution containing CaCl<sub>2</sub> is mixed with
Q27: A geological sample is found to have
Q31: Determine the identity of the daughter nuclide
Q50: Use the standard half-cell potentials listed below
Q72: Consider the following reaction, equilibrium concentrations, and
Q76: A particular first-order reaction has a rate
Q89: Determine the Q of a reaction at
Q91: Determine the identity of the daughter nuclide
Q117: The equilibrium constant is given for one