What is the pH of a solution made by mixing 30.00 mL of 0.10 mol L-1 acetic acid (CH3COOH) with 50.00 mL of 0. 100 mol L-1 KOH? Assume that the volumes of the solutions are additive. Ka = 1.8 × 10-5 for 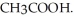
Definitions:
Federal Unemployment Tax
A payroll tax paid by employers based on the wages paid to employees, used to fund state workforce agencies.
FICA Tax
Federal Insurance Contributions Act tax, a U.S. federal payroll tax imposed on both employees and employers to fund Social Security and Medicare.
Federal Income Tax
A tax levied by the federal government on the annual income of individuals, corporations, trusts, and other legal entities.
Payroll Tax Expense
Taxes imposed on employers and employees, calculated as a percentage of salaries paid.
Q1: Which nuclide below is most likely to
Q20: The splitting of the uranium atom is
Q36: Do both protons ionize instantaneously from a
Q46: Calculate Δ<sub>r</sub>G° at 298 K using the
Q55: Calculate Δ<sub>r</sub>G at 298 K under the
Q86: Why are iron nails coated with zinc?
Q120: Determine the [OH⁻] concentration in a 0.235
Q130: Calculate the frequency factor, A, for a
Q135: Which of the following is the correct
Q135: Calculate the activation energy, E<sub>a</sub>, for a