Determine the ammonia concentration of an aqueous solution that has a pH of 11. 00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 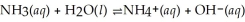
Definitions:
Perpetrators
Individuals who commit harmful, illegal, or unethical acts.
Contextual Influence
The impact that surrounding circumstances, environments, or conditions have on individuals' perceptions, behaviors, and decisions.
Career Choice
The process of deciding on a specific path or direction for one's professional life, often influenced by personal interests, values, and external factors.
Nontraditional Students
Individuals who pursue tertiary education at an older age than typical undergraduates, often juggling education with other responsibilities like work or family.
Q8: Which of the following should have the
Q53: Determine the value of K<sub>c</sub> for the
Q55: Consider the following reaction: NO(g) + SO<sub>3</sub>(g)
Q71: Calculate the concentration of bicarbonate ion, HCO<sub>3</sub><sup>-</sup>,
Q98: A KCl solution is prepared by dissolving
Q108: Which one of the following statements is
Q121: Given the following rate law, how does
Q125: For the following example, what is true
Q141: What is the molar solubility of AgCl
Q153: A 100.0 mL sample of 0.180 mol