Determine the ammonia concentration of an aqueous solution that has a pH of 11. 00. The equation for the dissociation of NH3 (Kb = 1.8 × 10-5) is below: 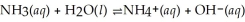
Definitions:
Horizontal Axis
A graph's base line for plotting and reading values, typically representing time or categories.
Operating Leverage
Operating leverage refers to the degree to which a company can increase operating income by increasing sales, highlighting the impact of fixed versus variable costs.
Operating Income
The profit realized from a business's operational activities, calculated before interest and taxes.
Operating Leverage
A measure of how revenue growth translates into growth in operating income, indicating the degree to which a company can increase profit by increasing sales.
Q9: Define allotrope.
Q17: Identify the classification of opal.<br>A) aerosol<br>B) solid
Q18: What is the pH of pure water
Q18: The boiling point elevation of an aqueous
Q21: What happens to the concentration of reactants
Q44: The rate constant for a second-order reaction
Q69: A 1.0 L buffer solution is 0.050
Q91: The boiling point of an aqueous 1.83
Q117: Given the following proposed mechanism, predict the
Q160: A reaction is found to have a