Express the equilibrium constant for the following reaction: 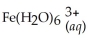 + 6CN-(aq) ⇌
+ 6CN-(aq) ⇌ 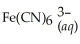 + 6H2O(l)
+ 6H2O(l)
Definitions:
Letter Grades
Symbols used in educational systems to represent the quality of a student's performance.
Median
The middle value in a data set when the values are arranged in ascending or descending order, or the average of two middle numbers if the dataset has an even number of observations.
Set of Data
A collection of related pieces of information or numerical values subject to analysis.
Mean
The central value of a numeric dataset, determined by summing all the numbers and dividing by the total number of values present.
Q19: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS, AgCl, NH<sub>4</sub>Cl,
Q31: Which of the following compounds is highly
Q62: Consider the following reaction at constant pressure.
Q62: pH = pK<sub>a2</sub><br>A)equivalence point of a weak
Q77: Calculate Δ<sub>r</sub>G° at 298 K using the
Q87: Which of the following is an acid-base
Q102: Which of the following statements is TRUE?<br>A)
Q108: Identify a heterogeneous catalyst.<br>A) CFCs with ozone<br>B)
Q121: Why is the quantity of energy required
Q168: A reaction with an activation energy of