At 900.0 K, the equilibrium constant (Kp) for the following reaction is 0.345 bar-1: 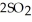 +
+ 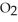 (g) →
(g) → 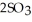 (g) At equilibrium, the partial pressure of
(g) At equilibrium, the partial pressure of 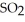 is 35.0 bar and that of
is 35.0 bar and that of 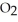 is 15.9 bar. The partial pressure of
is 15.9 bar. The partial pressure of 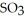 is ________ bar.
is ________ bar.
Definitions:
Price Wars
A competitive situation where retailers or manufacturers continuously lower prices to undercut competitors, often resulting in decreased profit margins.
Consolidation
The process of combining multiple accounts or businesses into a single aggregation for reporting and analysis purposes, or the decrease in a stock's price volatility and the formation of a clear resistance or support level after a significant price movement.
Cyclical Industries
Industries whose performance is heavily influenced by the economic cycle, experiencing higher sales during economic booms and lower sales during recessions.
Sell Government Bonds
The act of disposing of government debt securities in the financial market.
Q54: Which of the following is TRUE if
Q67: Express the equilibrium constant for the following
Q68: Which of the following is TRUE?<br>A) A
Q85: Express the equilibrium constant for the following
Q88: Consider the following reaction, equilibrium concentrations, and
Q106: Which of the following statements is TRUE?<br>A)
Q111: Determine the molar solubility of MgCO<sub>3 </sub>in
Q138: The isomerization of methylisonitrile to acetonitrile <img
Q142: Consider the following reaction and its equilibrium
Q150: Determine the vapour pressure of a solution