The decomposition of 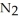
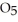 in solution in carbon tetrachloride proceeds via the reaction 2
in solution in carbon tetrachloride proceeds via the reaction 2 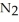
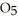 (soln) →
(soln) → 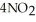 (soln) +
(soln) + 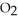 (soln)
(soln)
The reaction is first order and has a rate constant of 4.82 × 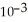
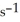 at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
Definitions:
Divisor Zero
Refers to the attempt of dividing a number by zero, which is undefined and causes an error in many programming environments.
Negative Value
A numerical value less than zero, representing the opposite of positive values.
Exception Class
A type of class in object-oriented programming that describes an error or unexpected event that can occur during the execution of a program.
Q20: Nitrogen dioxide decomposes at 300 °C via
Q26: Which one of the following will form
Q49: Consider the following reaction at equilibrium. What
Q51: Determine the molecular geometry for the molecule
Q53: Identify the triprotic acid.<br>A) HNO<sub>3</sub><br>B) H<sub>3</sub>PO<sub>4</sub><br>C) H<sub>2</sub>SO<sub>3</sub><br>D)
Q59: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="The for
Q60: Choose the compound below that contains at
Q118: Can the K<sub>p</sub> and K<sub>c</sub> for a
Q119: A reaction with an activation energy of
Q135: Draw a molecular orbital diagram and use