If a reaction carried out at 61 °C has an activation energy, Ea, of 67.3 kJ mol-1, a rate constant of 791 s-1, and a frequency factor, A, of 2.65 × 1013, what is the value of R? k = A 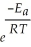
Definitions:
Long-run Real Wages
The inflation-adjusted income received by workers, taking into account the purchasing power of their earnings over a long period.
Demand for Labor
The total amount of workforce or labor hours that employers in the economy are willing and able to hire at a given wage rate.
Supply of Labor
The total hours that workers wish to work at a given wage rate, reflecting how many people are available to work.
Nominal Wage
The amount of money received by a worker per unit of time (hour, day, etc.); money wage.
Q15: Which type of bonding does Sr form
Q50: A reaction with an activation energy of
Q53: Consider the molecule below. Determine the molecular
Q88: Place the following compounds in order of
Q97: If the activation energy for a given
Q121: Given the following rate law, how does
Q128: Express the equilibrium constant for the following
Q133: Calculate the frequency factor, A, for a
Q140: Place the following compounds in order of
Q158: Which of the following concentration units are