The decomposition of 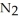
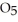 in solution in carbon tetrachloride proceeds via the reaction 2
in solution in carbon tetrachloride proceeds via the reaction 2 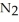
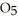 (soln) →
(soln) → 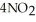 (soln) +
(soln) + 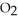 (soln)
(soln)
The reaction is first order and has a rate constant of 4.82 × 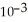
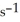 at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
at 64 °C. If the reaction is initiated with 0.073 mol in a 1.00 L vessel, how many moles remain after 151 s?
Definitions:
Tap Water
Water supplied to a residential or commercial area through a system of pipes and taps, generally suitable for drinking and other domestic purposes.
Diode Trio
A set of three diodes used in an alternator to convert AC to DC current, essential for charging a vehicle's battery.
Loose Belt
A belt in a mechanical system that lacks proper tension, potentially leading to slippage, reduced efficiency, or damage to equipment.
Electrolyte
A substance that produces an electrically conducting solution when dissolved in a polar solvent, such as water, enabling it to conduct electricity.
Q5: What is the pH of the resulting
Q9: Which of the following statements is TRUE?<br>A)
Q31: Which of the following compounds is highly
Q41: What is the unit of k in
Q64: Place the following compounds in order of
Q77: Which of the following represents the integrated
Q101: What is the activation energy for a
Q111: What is the conjugate acid of HCO<sub>3</sub>⁻
Q147: Draw a molecular orbital diagram and use
Q155: Determine the electron geometry (eg) and molecular