A solution is prepared by dissolving 16.4 g of benzene ( 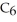
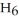 ) in 282 g of carbon tetrachloride
) in 282 g of carbon tetrachloride 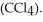 The concentration of benzene in this solution is ________ molal. The molar masses of
The concentration of benzene in this solution is ________ molal. The molar masses of 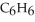 and
and 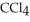 are
are 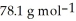 and
and 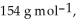 respectively.
respectively.
Definitions:
Portable Benefits
Benefits, such as retirement plans or health insurance, that can be retained by employees even if they change jobs.
Long-term Care Insurance
A type of insurance designed to cover costs associated with long-term care services, such as in-home care, assisted living, or nursing home care.
401(k) Plan
A retirement savings plan sponsored by an employer that allows workers to save and invest a piece of their paycheck before taxes are taken out.
Paid Leave
Time off from work that is compensated, including but not limited to vacation, sick leave, and parental leave.
Q7: Describe a sigma bond.<br>A) p orbital overlapping
Q59: At 20 °C, a 0.389 mol L<sup>-1</sup>
Q69: What is the strongest type of intermolecular
Q77: Which of the following represents the integrated
Q79: A small amount, 6.78 g, of pentane
Q89: What volume of a 0.716 mol L<sup>-1</sup>
Q100: Describe a molecule that can be a
Q120: The rate constant, k, for a first-order
Q130: How many of the following molecules are
Q160: A reaction is found to have a