At 20 °C, a 0.389 mol L-1 aqueous solution of ammonium chloride has a density of 1.0064 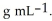 What is the mass % of ammonium chloride in the solution? The formula weight of
What is the mass % of ammonium chloride in the solution? The formula weight of 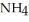 Cl is
Cl is 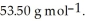
Definitions:
Bond Discount
The difference between the face value of a bond and the price for which it is sold, when the bond is issued at less than its face value.
Equity Method
A method where an investment is initially recorded at cost and subsequently adjusted for the investor's proportionate share in the net assets of the investee, recognizing income or loss.
Intercompany Sale
Transactions that occur between two divisions or subsidiaries within the same parent company, often for the purpose of transferring goods or services.
Pre-tax Gain
Income generated before any taxes have been applied.
Q31: What is the unit of k in
Q35: Give the edge length in terms of
Q42: The following reaction is exothermic. Which change
Q50: A solution is prepared by dissolving 21.0
Q73: Of the following elements, which has the
Q76: Consider the molecule below. Determine the hybridization
Q105: A molecule containing a central atom with
Q129: Identify the shortest bond.<br>A) single covalent bond<br>B)
Q143: Determine the electron geometry for the molecule
Q171: What is the O-B-O bond angle in