An aqueous solution has a normal boiling point of 10 3.0 °C. What is the freezing point of this solution? For water 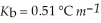 and
and 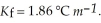
Definitions:
Intra-entity Gross Profit
The profit recognized from transactions within the same company, affecting consolidated financial statements.
Eliminate Unrecognized
The process of removing gains, losses, or other items that have not been realized or acknowledged formally from the financial statements.
Intra-entity Gross Profit
The profit recorded from transactions that occur within the same entity, often eliminated in consolidation.
Eliminate Intra-entity Transfer
The process of removing sales and purchases of goods or services made between companies within the same corporate group in consolidated financial statements.
Q16: Give the coordination number for a body-centred
Q40: The isomerization of methylisonitrile to acetonitrile <img
Q84: Calculate the solution temperature required to produce
Q104: Calculate the freezing point of a solution
Q118: Choose the bond below that is most
Q127: Choose the situation below that would result
Q130: How many of the following molecules are
Q142: What is the unit of k in
Q144: Calculate the pH of a 0. 60
Q150: Determine the vapour pressure of a solution