A solution is prepared by dissolving 21.0 g of glycerin ( 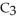
 ) in 201 g of ethanol
) in 201 g of ethanol 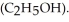 The freezing point of the solution is ________°C. The freezing point of pure ethanol is
The freezing point of the solution is ________°C. The freezing point of pure ethanol is 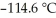 . The molal freezing point depression constant (
. The molal freezing point depression constant ( 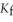 ) for ethanol is
) for ethanol is 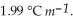 The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
The molar masses of glycerin and of ethanol are 92.1 g mol-1 and 46.1 g mol-1, respectively.
Definitions:
Disengagement Theory
Theory of aging that holds that successful aging is characterized by mutual withdrawal of the older person and society.
Activities
Actions or tasks that individuals engage in, often for purposes of leisure, recreation, or achieving specific goals.
Commitments
Promises or obligations that an individual has decided to carry out or fulfill.
Adaptive Defenses
Psychological strategies employed by individuals to manage emotions and stress, often unconsciously, in a way that improves emotional well-being.
Q4: The enthalpy change for converting 1.00 mol
Q29: Identify the strongest bond.<br>A) single covalent bond<br>B)
Q46: In a reaction mixture containing reactants and
Q48: Ethanol ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Ethanol (
Q54: What is the implication of Q <
Q58: Place the following in order of decreasing
Q67: Express the equilibrium constant for the following
Q83: A particular first-order reaction has a rate
Q104: Without performing complex calculations, determine the order
Q163: Which of the following statements is FALSE?<br>A)