Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water. The molal freezing point depression constant ( 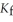 ) for water is
) for water is 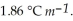
Definitions:
Objects
In programming and software, refers to instances of a class that encapsulate data and functionality for manipulation of that data.
Transition Speed
The duration it takes for a slide, scene, or element to move from one state to another in multimedia and web applications.
Duration
The amount of time that something lasts or continues.
Shape Outline
The external line or border that defines the form of an object in graphics software.
Q7: Which of the following substances would you
Q20: Nitrogen dioxide decomposes at 300 °C via
Q32: Which of the following statements is TRUE?<br>A)
Q32: How much energy must be removed from
Q47: Place the following in order of increasing
Q55: t<sub>1/2 </sub><br>A)reaction order<br>B)frequency factor<br>C)activation energy<br>D)rate constant<br>E)half-life
Q73: An unknown nonelectrolyte and nonvolatile compound is
Q74: What is the molality of a glucose
Q89: sp<br>A)sp hybridized central atom<br>B)octahedral electron geometry<br>C)polar, but
Q140: How many grams of KBr are required