At 20 °C, an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 1.0674 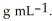 What is the molarity of ammonium chloride in the solution? The formula weight of
What is the molarity of ammonium chloride in the solution? The formula weight of 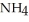 Cl is
Cl is 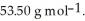
Definitions:
Taste Aversions
The acquired aversion to specific food or beverage due to its connection with a distressing or sickening event.
Unconditioned Stimulus
In classical conditioning, a stimulus that naturally and automatically triggers a response without any need for learning.
Learned By Association
The process of forming connections between stimuli and responses, typically through conditioning.
Ivan Pavlov
A Russian physiologist known for his work in classical conditioning, demonstrating how stimuli can trigger conditioned responses in dogs.
Q11: Define osmosis.
Q26: Carbon-14, which is present in all living
Q45: Consider the following reaction, equilibrium concentrations, and
Q53: The forces between polar molecules is known
Q57: Which of the following resonance structures for
Q72: Use molecular orbital theory to determine whether
Q79: K<sub>c</sub> is 1.67 × 10<sup>20</sup> mol<sup>3</sup> L<sup>-3</sup>
Q92: Choose the solvent below that would show
Q133: Calculate the frequency factor, A, for a
Q143: What is the hydroxide ion concentration of