Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water. The molal freezing point depression constant ( 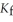 ) for water is
) for water is 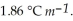
Definitions:
Working Capital
The difference between a company's current assets and current liabilities, indicating the liquidity available for running day-to-day operations.
Annual Cost Savings
The reduction in expenses that a company achieves within one year through cost-cutting measures or process improvements.
Pretax Return
The income or profit that a company generates before any taxes have been applied.
Discount Factor(s)
A mathematical factor used to calculate the present value of a future amount of money or stream of cash flows.
Q15: The aquation of tris (1,10-phenanthroline)iron(II) in acid
Q32: Choose the statement below that is TRUE.<br>A)
Q60: Given the following balanced equation, determine the
Q62: Determine the molecular geometry for the molecule
Q68: Explain why oil and water do not
Q81: Draw the Lewis structure for the molecule
Q92: For which of the following reactions will
Q115: How many of the following molecules contain
Q149: To make a 2.00 mol kg<sup>-1</sup> solution,
Q166: Determine the vapour pressure of a solution