Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 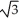 .
.
Definitions:
Glia
Non-neuronal cells in the nervous system that provide support and protection for neurons, playing critical roles in brain function.
Electrical Charge
A physical property of matter that causes it to experience a force when placed in an electromagnetic field.
Millivolts
A unit of electric potential equal to one-thousandth of a volt, commonly used in measuring small voltage differences.
Neuron
A specialized cell in the nervous system that transmits information to other nerve cells, muscle cells, or gland cells, primarily through electrical and chemical signals.
Q14: The first-order reaction, SO<sub>2</sub>Cl<sub>2</sub> → SO<sub>2</sub> +
Q19: Using the VSEPR model, the molecular geometry
Q31: The phosphorus atom in PCl<sub>3</sub> would be
Q36: How many lone pairs are on the
Q38: Use the data given below to construct
Q44: The rate constant for a second-order reaction
Q44: A 1.00 L sample of water contains
Q51: Place the following ions in order of
Q66: Calculate the mole fraction of total ions
Q139: How much water must be added to