Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) . The specific heat of CCl4(l) is 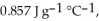 its heat of fusion is
its heat of fusion is 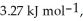 and its heat of vaporization is
and its heat of vaporization is 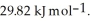
Definitions:
Blueberries
A type of perennial flowering plants with blue or purple berries, known for their nutritional benefits and antioxidants.
Bear
A large mammal belonging to the Ursidae family, known for its omnivorous diet, thick fur, and powerful physique.
Producer
An organism, usually a plant or bacterium, that can produce its own food through photosynthesis or chemosynthesis, forming the basis of the food chain.
Ecosystem
A group of living beings interacting with the abiotic elements of their surrounding, functioning as a cohesive system.
Q3: Identify the type of solid for argon.<br>A)
Q9: In the best Lewis structure for CN<sup>-</sup>,
Q16: How many of the following molecules have
Q26: Identify the type of solid for diamond.<br>A)
Q35: Give the edge length in terms of
Q89: What volume of a 0.716 mol L<sup>-1</sup>
Q105: Identify the compound with covalent bonding.<br>A) Li<sub>2</sub>S<br>B)
Q129: Consider the following reaction at equilibrium. What
Q155: What mass of ethane (CH<sub>3</sub>CH<sub>3</sub>) is contained
Q161: Draw a molecular orbital diagram and use