Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) . The specific heat of CCl4(l) is 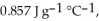 its heat of fusion is
its heat of fusion is 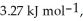 and its heat of vaporization is
and its heat of vaporization is 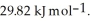
Definitions:
Ammonia Molecules
Consists of one nitrogen and three hydrogen atoms, NH3, a colorless gas with a distinctive pungent smell, used as a fertilizer and in many industrial processes.
Poisoned
A condition caused by ingesting, inhaling, or coming into contact with toxic substances.
Ionic Compound
A substance made up of ions that are bonded together by the force of electrostatic attraction, known as ionic bonding.
Thallium(I) Nitrate
A chemical compound with the formula TlNO3, used in the manufacture of special glasses and in chemical synthesis.
Q20: Nitrogen dioxide decomposes at 300 °C via
Q30: Which of the following reactions is associated
Q43: How many valence electrons do the alkaline
Q43: If the concentration of a reactant is
Q61: Identify the ground-state electron configuration for Cd.<br>A)
Q71: The normal boiling point of water is
Q73: An unknown nonelectrolyte and nonvolatile compound is
Q95: Place the following substances in order of
Q114: Choose the best Lewis structure for PO<sub>4</sub><sup>3</sup>⁻.<br>A)
Q124: Given the following rate law, how does