Multiple Choice
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ. The specific heats of ice, water, and steam are 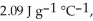
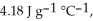 and
and 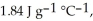 respectively. For
respectively. For 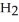 O,
O, 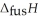 = 6.01 kJ mol-1, and
= 6.01 kJ mol-1, and 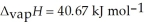 .
.
Definitions:
Related Questions
Q34: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt=" decomposes
Q62: A solution of methanol and water has
Q94: Determine the molecular geometry for the molecule
Q107: How many moles of KF are contained
Q119: Calculate the solution temperature required to produce
Q124: A mixture of pentane and hexane has
Q138: A solution is formed at room temperature
Q142: What is the unit of k in
Q157: Identify the classification of whipped cream.<br>A) aerosol<br>B)
Q165: Use the molecular orbital diagram shown to