Using the following equation for the combustion of octane, calculate the heat of reaction for 100.0 g of octane. The molar mass of octane is 114.33 g 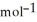 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 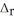 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Prospective Client
An individual or organization that is considered a potential customer and may be interested in the company's products or services.
Payment Options
Various methods available to pay for goods or services, including cash, credit cards, and electronic payments.
Growing Annuity
A growing annuity is a type of annuity payment that increases at a constant rate over time, often used to model increasing cash flows like rent or royalties.
Fixed Period
A specified duration of time during which certain conditions or rules are applied, often in financial contracts or investment terms.
Q6: Define pressure.
Q13: Which ionization process requires the most energy?<br>A)
Q39: What is the effective nuclear charge experienced
Q55: Identify the element that has a ground-state
Q56: Determine the oxidizing agent in the following
Q68: A compound is found to be 30.45%
Q75: Identify the set of four quantum numbers
Q121: Rank the following molecules in decreasing bond
Q146: Which of the following will cause the
Q151: Which of the following statements is FALSE?<br>A)