How much energy is evolved during the reaction of 48.7 g of Al according to the reaction below? Assume that there is excess
Fe2O3. Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 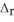 H° = -852 kJ
H° = -852 kJ
Definitions:
Assets
Resources owned by a company that have economic value and can be used to meet its future obligations.
Liabilities
are legal financial debts or obligations that arise during the course of business operations.
Owner's Equity
The residual interest in the assets of a business after deducting liabilities, representing the ownership interest of shareholders or partners.
Liabilities
Financial obligations or debts owed by a business to outsiders, such as loans, accounts payable, or mortgages.
Q12: An element that has the valence electron
Q38: What is the volume of 9.783 ×
Q48: Ideal gas law<br>A)PV = nRT<br>B)V<sub>1</sub>/T<sub>1</sub> = V<sub>2</sub>/T<sub>2</sub><br>C)V<sub>1</sub>/n<sub>1</sub>
Q48: Calculate the orbital for the hydrogen atom
Q69: Calculate the amount (mass) of acetaldehyde (C<sub>2</sub>H<sub>4</sub>O,
Q72: A piece of gold with a mass
Q113: The specific heat capacity of solid copper
Q133: Choose the valence orbital diagram that represents
Q219: How many grams of CH<sub>3</sub>OH must be
Q243: Balance the following redox reaction if it