How much energy is evolved during the formation of 98.7 g of Fe according to the reaction below?
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 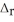 H° = -852 kJ
H° = -852 kJ
Definitions:
Situational Leadership
A leadership style that suggests the best leaders adjust their leadership approach based on the maturity and capability of their followers or the specifics of the situation.
Subordinate Needs
The requirements or expectations that employees at lower hierarchical levels within an organization have from their work and workplace.
Leadership Styles
The various approaches and methods leaders use to motivate, guide, and manage teams and organizations.
Telephone
A communications device that allows two or more users to conduct a conversation when they are too far apart to be heard directly.
Q13: Determine the limiting reactant (LR) and the
Q42: Which of the following gases has the
Q52: Choose the best Lewis structure for NO<sub>3</sub>⁻.<br>A)
Q73: Determine the volume of H<sub>2</sub>S (at 375
Q111: Choose the best Lewis structure for SF<sub>4</sub>.<br>A)
Q127: Which molecule or compound below contains an
Q129: Place the following in order of decreasing
Q136: Se-I<br>A)strongest covalent bond<br>B)longest covalent bond<br>C)weakest ionic bond<br>D)metallic
Q145: Balance the following redox reaction if it
Q238: How many moles of NaCl are required