Using the following thermochemical equation, determine the amount of heat produced per kg of CO2 formed during the combustion of benzene (C6H6) . 2C6H6(l) + 15O2(g) → 12CO2(g) + 6H2O(g) 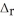 H° = -6278 kJ
H° = -6278 kJ
Definitions:
Full-Ride Scholarship
A scholarship that covers all expenses of a student's education, including tuition, room, board, and sometimes additional costs like books and fees.
Probability
The measure of the likelihood that an event will occur, expressed as a number between 0 and 1.
Life Expectancy
The average period that a person is expected to live, which can vary significantly across different countries and communities.
Inflation
The rate at which the general level of prices for goods and services is rising, and, subsequently, purchasing power is falling.
Q30: Identify the ground-state electron configuration for Sr.<br>A)
Q39: Which of the following occurs as the
Q58: The specific heat capacity of methane gas
Q63: How many molecules of XeF<sub>6 </sub>are formed
Q86: Calculate the initial temperature of 448 g
Q88: Choose the best Lewis structure for ICl<sub>5</sub>.<br>A)
Q137: Use the periodic table to predict the
Q148: Which of the following compounds is an
Q155: 8.000 moles of H<sub>2</sub>(g) reacts with 4.000
Q223: A student prepared a stock solution by