Using the following equation for the combustion of octane, calculate the amount of moles of carbon dioxide formed from 100.0 g of octane. The molar mass of octane is 114.33 g  . The molar mass of carbon dioxide is 44.0095 g
. The molar mass of carbon dioxide is 44.0095 g 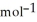 . 2C8H18 + 25O2 → 16CO2 + 18H2O
. 2C8H18 + 25O2 → 16CO2 + 18H2O 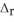 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Profitability
The ability of a company to generate earnings compared to its expenses and other relevant costs incurred during a specific period of time.
Transactional Leaders
Leaders who focus on the role of supervision, organization, and group performance; they are concerned with status quo and day-to-day progress toward goals, often employing a reward-punishment system.
Employee Satisfaction
The level of contentment employees feel regarding their job and workplace conditions, which can impact productivity and loyalty.
Bartering
A system of exchange in which participants in a transaction directly trade goods or services without using a medium of exchange, such as money.
Q52: Determine the mass of a ball with
Q61: Which of the following ionic compounds would
Q78: Use the standard reaction enthalpies given below
Q82: What was the predicted formula for the
Q105: For a hydrogen atom, which electronic transition
Q118: How many millilitres of a 0.266 mol
Q135: A 50.0 g sample of liquid water
Q142: Using the periodic table, identify the element
Q170: Choose the paramagnetic species from below.<br>A) Ti<sup>4</sup>⁺<br>B)
Q204: Aluminum metal reacts with aqueous iron( II)