A 100.0 mL sample of 0.300 mol L-1 NaOH is mixed with a 100.0 mL sample of 0.300 mol L-1 HNO3 in a coffee cup calorimeter. If both solutions were initially at 35.00 °C and the temperature of the resulting solution was recorded as 37.00 °C, determine the 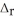 H° (in units of kJ
H° (in units of kJ 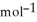 ) for the neutralization reaction between aqueous NaOH and HNO3 . Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
) for the neutralization reaction between aqueous NaOH and HNO3 . Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
Definitions:
Zildjian Company
A manufacturer known for producing cymbals and drumsticks, recognized as one of the oldest companies in the world.
Company Legacy
The lasting impression and impact left by a company on its industry, community, and stakeholders, often associated with its brand, values, or contributions.
Interdisciplinary Nature
Refers to approaches or studies that integrate knowledge and methods from various disciplines to address complex problems or create new understanding.
Information Explosion
Refers to the rapid increase and availability of information, primarily due to digital advancements, resulting in an overwhelming volume of data to process.
Q17: A piece of uranium with a mass
Q29: Determine the oxidation state of S in
Q60: Calculate the wavelength of an electron (m
Q61: Identify the ground-state electron configuration for Cd.<br>A)
Q73: Determine the mass of a ball with
Q120: What are the required coefficients to properly
Q124: Sodium metal and water react to form
Q127: Why are the standard heats of formation
Q140: What pressure will 2.6 × 10<sup>23</sup> molecules
Q178: Balance the chemical equation given below, and