Two solutions, initially at 24.69 °C, are mixed in a coffee cup calorimeter (Ccal = 105.5 J °C-1) . When a 200.0 mL volume of 0.100 mol L-1 AgNO3 solution is mixed with a 100.0 mL sample of 0.100 mol L-1 NaCl solution, the temperature in the calorimeter rises to 25.16 °C. Determine the 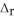 H°, in units of kJ
H°, in units of kJ 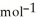 . Assume that the density and heat capacity of the solutions is the same as that of water. Hint: Write a balanced reaction for the process.
. Assume that the density and heat capacity of the solutions is the same as that of water. Hint: Write a balanced reaction for the process.
Definitions:
Plant Species
A category that classifies a group of plants which are similar and capable of interbreeding.
Biodiversity
The variety and variability of life on Earth, including diversity within species, between species, and of ecosystems.
Excessive Ultraviolet Radiation
Overexposure to UV rays from the sun or artificial sources, which can lead to skin damage and increased risk of skin cancer.
Ultraviolet Radiation
A form of energy emitted by the sun, invisible to the naked eye, which can have harmful effects on human skin and eyes but is essential for the synthesis of vitamin D.
Q6: What is the effective nuclear charge experienced
Q80: The density of a gas is 1.41
Q98: Choose the paramagnetic species from below.<br>A) Ca<br>B)
Q118: How many millilitres of a 0.266 mol
Q123: Place the following elements in order of
Q144: According to the following balanced reaction, how
Q145: Place the following elements in order of
Q149: How many photons are contained in a
Q163: Which of the following quantum numbers describes
Q230: Which of the following pairs of aqueous