Multiple Choice
Use the standard reaction enthalpies given below to determine 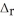 H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g)
H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g) 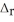 H° = ?
H° = ?
Given:
SO2(g) → S(s) + O2(g) 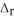 H° = +296.8 kJ
H° = +296.8 kJ
2SO2(g) + O2(g) → 2SO3(g) 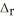 H° = -197.8 kJ
H° = -197.8 kJ
Understand the principles and application of time-driven activity-based costing.
Perform Customer Cost Analysis using time-driven activity-based costing.
Perform Capacity Analysis using time-driven activity-based costing to evaluate expense impacts of matching capacity with demand.
Analyze and interpret the financial data specific to Customer Service, Order Fulfillment, and Tech Support Departments.
Definitions:
Related Questions
Q21: Which of the following occurs as the
Q31: What are the possible orbitals for n
Q33: For n = 3, what are the
Q39: Calculate the amount (mass) of acetic acid
Q44: Define diffraction.
Q46: Hydrogen peroxide decomposes to water and oxygen
Q50: What is the oxidation number change for
Q130: Identify the reducing agent in the following
Q151: Which of the following statements is FALSE?<br>A)
Q186: Balance the chemical equation given below, and