The specific heat capacity of liquid mercury is 0.14 J 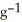
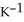 . How many joules of heat are needed to raise the temperature of 4.00 g of mercury from 19.0°C to 39.5°C?
. How many joules of heat are needed to raise the temperature of 4.00 g of mercury from 19.0°C to 39.5°C?
Definitions:
Currency R Strengthens
The condition where Currency R increases in value relative to other currencies, leading to increased purchasing power internationally.
Exchange Rate
The value assigned to one currency when trading for another.
Units
A fundamental quantity used in mathematics and science to measure variables.
C$ Weakens
A situation in which the Canadian dollar decreases in value relative to other currencies.
Q5: What was the predicted formula for the
Q5: In which orbital below would an electron
Q11: Describe the difference between complete ionic and
Q25: Determine the total volume of all gases
Q46: What is the maximum number of p
Q94: Which of the following transitions (in a
Q101: An 8.21 g sample of glycerol (C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>,
Q106: Calculate the energy of the violet light
Q108: Calculate the kinetic energy of a 150
Q168: Which of the following statements is FALSE?<br>A)