How much heat is absorbed when 50.00 g of C(s) reacts in the presence of excess SO2(g) to produce CS2(l) and CO(g) according to the following chemical equation?
5C(s) + 2SO2(g) → CS2(l) + 4CO(g) 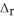 H° = 239.9 kJ
H° = 239.9 kJ
Definitions:
Industry Supply Curve
A graphical representation showing the total quantity of a good or service that producers in an industry are willing and able to supply at different price levels.
Marginal Cost Curves
A graphical representation showing how the cost of producing one more unit of a good varies with the quantity of the good produced.
AVC
Average Variable Cost, which is the total variable costs divided by the quantity of output produced.
Average Variable Cost
The total variable costs (costs that change with production volume) divided by the quantity of output produced.
Q5: How many electrons are transferred in the
Q33: If a reaction is carried out at
Q53: Which of the following is a molecular
Q66: Give the ground-state electron configuration for Br⁻.<br>A)
Q73: Determine the volume of H<sub>2</sub>S (at 375
Q80: Identify the ground-state electron configuration for Se.<br>A)
Q84: Place the following gases in order of
Q85: A mixture of 0.220 moles CO, 0.350
Q100: Use the information provided to determine <img
Q236: Identify the balanced equation to show the